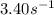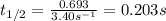Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 23.06.2019 16:50
How can a scientist assess whether a pure niobium (nb) sample is responsible for contaminating the lab with radioactivity? test the niobium sample to see whether it now contains other elements.test the niobium sample for the presence of niobium oxide compounds.heat the niobium, and see if the level of radioactivity in the lab increases.place the niobium under pressure, and see if the level of radioactivity in the lab increases.
Answers: 3

Chemistry, 23.06.2019 19:00
Explain how scientific advancements have lessened the effects of hazardous events on society.
Answers: 1
You know the right answer?
The rate constant for the first-order decomposition of n2o is 3.40 s-1. what is the half-life of the...
Questions

Arts, 05.02.2021 01:50

Mathematics, 05.02.2021 01:50

Mathematics, 05.02.2021 01:50


English, 05.02.2021 01:50

Chemistry, 05.02.2021 01:50


Mathematics, 05.02.2021 01:50


Mathematics, 05.02.2021 01:50



Mathematics, 05.02.2021 01:50

History, 05.02.2021 01:50

Biology, 05.02.2021 01:50



Biology, 05.02.2021 01:50

Mathematics, 05.02.2021 01:50

Physics, 05.02.2021 01:50

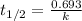
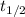 = half-life of the reaction
= half-life of the reaction