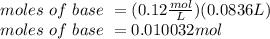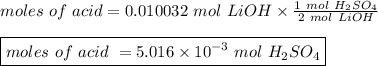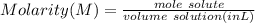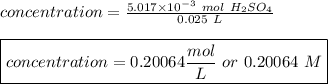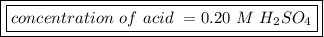Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
The titration of 25.0 ml of an unknown concentration h2so4 solution requires 83.6 ml of 0.12 m lioh...
Questions

Business, 09.03.2020 00:45

Business, 09.03.2020 00:45

Mathematics, 09.03.2020 00:45

History, 09.03.2020 00:45


Social Studies, 09.03.2020 00:46

Mathematics, 09.03.2020 00:46






Mathematics, 09.03.2020 00:47

Mathematics, 09.03.2020 00:47

History, 09.03.2020 00:47

Mathematics, 09.03.2020 00:47

Social Studies, 09.03.2020 00:47

History, 09.03.2020 00:47

Mathematics, 09.03.2020 00:48

Mathematics, 09.03.2020 00:48