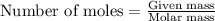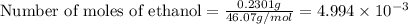Chemistry, 11.10.2019 18:40 brydenskl814
Asample of ethanol (c2h6o) has a mass of 0.2301 g. complete combustion of this sample causes the temperature of a bomb calorimeter to increase by 1.33°c. the calorimeter has a mass of 2.000 kg and a specific heat of 2.45 j/g•°c. how many moles of ethanol are present in the sample?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
Asample of ethanol (c2h6o) has a mass of 0.2301 g. complete combustion of this sample causes the tem...
Questions

Social Studies, 24.07.2019 07:00


Social Studies, 24.07.2019 07:00

Mathematics, 24.07.2019 07:00

History, 24.07.2019 07:00

Mathematics, 24.07.2019 07:00



History, 24.07.2019 07:00


Mathematics, 24.07.2019 07:00

English, 24.07.2019 07:00

Social Studies, 24.07.2019 07:00

Arts, 24.07.2019 07:00

History, 24.07.2019 07:00




Mathematics, 24.07.2019 07:00

 moles
moles of particles.
of particles.