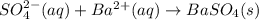Chemistry, 18.09.2019 23:30 cristal2000amber
What are the net ionic equations for:
ni(no3)2(aq) + na2s(aq) = nis(s) + 2 nano3(aq)
kbr(aq) + nano3(aq) = kno3(s) + nabr(aq)
li2so4(aq) + bacl2(aq) = baso4(s) + 2 licl(aq)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:10
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
What are the net ionic equations for:
ni(no3)2(aq) + na2s(aq) = nis(s) + 2 nano3(aq)
kb...
ni(no3)2(aq) + na2s(aq) = nis(s) + 2 nano3(aq)
kb...
Questions




Mathematics, 06.03.2021 08:10

Mathematics, 06.03.2021 08:10


Mathematics, 06.03.2021 08:10

English, 06.03.2021 08:10

Mathematics, 06.03.2021 08:10


Mathematics, 06.03.2021 08:10

Mathematics, 06.03.2021 08:10



English, 06.03.2021 08:10


Chemistry, 06.03.2021 08:10



Arts, 06.03.2021 08:10

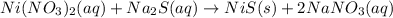
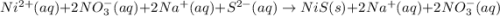
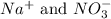 are the spectator ions.
are the spectator ions.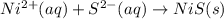
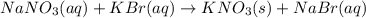
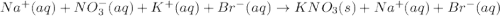
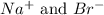 are the spectator ions.
are the spectator ions.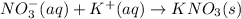
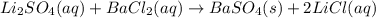
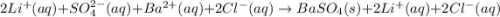
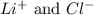 are the spectator ions.
are the spectator ions.