
Chemistry, 02.01.2020 10:31 frandariusscott
In the process of ionization, what is the relationship between the second ionization energy (i2) and the third ionization energy (i3)? i2 > i3 i2 < i3 i2 = i3 there is no way to predict this relationship.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2
You know the right answer?
In the process of ionization, what is the relationship between the second ionization energy (i2) and...
Questions

Chemistry, 26.06.2019 07:30


History, 26.06.2019 07:30



Physics, 26.06.2019 07:30

Health, 26.06.2019 07:30

Mathematics, 26.06.2019 07:30

Mathematics, 26.06.2019 07:30



Chemistry, 26.06.2019 07:30

Biology, 26.06.2019 07:30

Mathematics, 26.06.2019 07:30






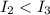
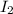 stands for second ionization energy means the energy required to remove second electron and
stands for second ionization energy means the energy required to remove second electron and 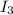 stands for third ionization energy means the energy required to remove the third electron.
stands for third ionization energy means the energy required to remove the third electron. 

