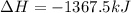Chemistry, 22.11.2019 08:31 aprilreneeclaroxob0c
Using the standard enthalpies of formation found in the textbook, determine the enthalpy change for the combustion of ethanol c2h5oh as given below. c2h5oh (l) + 3 o2(g) → 2 co2(g) + 3 h2o(g)

Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1

Chemistry, 23.06.2019 05:30
Scientist think that animals with remarkably similar embryo development probably shared a common ancestor
Answers: 1

You know the right answer?
Using the standard enthalpies of formation found in the textbook, determine the enthalpy change for...
Questions



Advanced Placement (AP), 14.09.2021 21:30


Mathematics, 14.09.2021 21:30


Mathematics, 14.09.2021 21:30


Mathematics, 14.09.2021 21:30

Mathematics, 14.09.2021 21:30



Mathematics, 14.09.2021 21:30

Mathematics, 14.09.2021 21:40

English, 14.09.2021 21:40

Mathematics, 14.09.2021 21:40



Advanced Placement (AP), 14.09.2021 21:40

![\Delta H=\sum [n\times H_f(product)]-\sum [n\times H_f(reactant)]](/tpl/images/0386/1954/c8099.png)
![\Delta H=[(n_{CO_2}\times H_f_{CO_2})+(n_{H_2O}\times H_f_{H_2O}) ]-[(n_{O_2}\times H_f_{O_2})+(n_{C_2H_5OH}\times H_f_{C_2H_5OH})]](/tpl/images/0386/1954/91c50.png)
![\Delta H=[(2\times -393.5 kJ/mol)+(3\times -285.5 k) ]-[(3\times 0)+(1\times -276]](/tpl/images/0386/1954/80382.png)