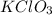Chemistry, 23.09.2019 11:30 nauticatyson9
A13.00 g sample of a compound contains 4.15 g potassium (k), 3.76 g chlorine (cl), and oxygen (o). calculate the empirical formula.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
A3.37-mg sample of protein was chemically digested to convert its nitrogen into ammonia and then diluted to 100.0 ml. then 10.0 ml of this solution was placed in a 50-ml volumetric flask and treated with 5 ml of phenol solution plus 2 ml of sodium hypochlorite solution. the sample was diluted to 50.0 ml, and the absorbance at 625 nm was measured in a 1.00-cm cuvette and found to be 0.486. for reference, a standard solution was prepared from 10.0 mg of nh4cl (molar mass = 53.49 grams/mole) dissolved in 1.00 l of water. then 10.0 ml of this standard was placed in a 50-ml volumetric flask, treated in the same manner as the unknown, and the absorbance found to be 0.323. finally, a reagent blank was prepared using distilled water in place of unknown, it was treated in the same manner as the unknown, and the absorbance found to be 0.076. calculate the weight percent of nitrogen in the protein.
Answers: 1

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
A13.00 g sample of a compound contains 4.15 g potassium (k), 3.76 g chlorine (cl), and oxygen (o). c...
Questions

Mathematics, 28.09.2019 10:10





Mathematics, 28.09.2019 10:10



Mathematics, 28.09.2019 10:10

Mathematics, 28.09.2019 10:10

Computers and Technology, 28.09.2019 10:10

Mathematics, 28.09.2019 10:10

Biology, 28.09.2019 10:10

Biology, 28.09.2019 10:10

History, 28.09.2019 10:10


Mathematics, 28.09.2019 10:10


Geography, 28.09.2019 10:10

History, 28.09.2019 10:10