Chemistry, 20.09.2019 01:00 kotetravels10
Asample of cacl2⋅2h2o/k2c2o4⋅h2o solid salt mixture is dissolved in ~150 ml de-ionized h2o. the oven dried precipitate has a mass of 0.333 g. the limiting reactant in the salt mixture is k2c2o4⋅h2o. cacl2⋅2h2o(aq) + k2c2o4⋅h2o(aq) à cac2o4⋅h2o(s) + 2kcl(aq) + 2h2o(l) starting material (sm) product molar mass (mm) g/mol: cacl2⋅2h2o = 147.02 k2c2o4⋅h2o = 184.24 cac2o4 = 128.10 determine mass of k2c2o4⋅h2o(aq) in salt mixture in grams. answer to 3 places after the decimal and include unit, g

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1

You know the right answer?
Asample of cacl2⋅2h2o/k2c2o4⋅h2o solid salt mixture is dissolved in ~150 ml de-ionized h2o. the oven...
Questions



Mathematics, 08.10.2021 08:00


Social Studies, 08.10.2021 08:00







Biology, 08.10.2021 08:00


History, 08.10.2021 08:00


Physics, 08.10.2021 08:00

Mathematics, 08.10.2021 08:00



Mathematics, 08.10.2021 08:00

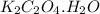 in the salt mixture is 0.424 grams.
in the salt mixture is 0.424 grams.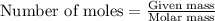 .....(1)
.....(1)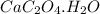 :
: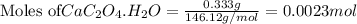
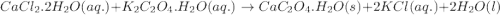
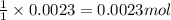 of
of