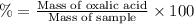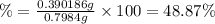Chemistry, 18.10.2019 10:30 anahitrejo1
Oxalic acid is a diprotic acid. calculate the percent of oxalic acid (h2c2o4) in a solid given that a 0.7984-g sample of that solid required 37.98 ml of 0.2283 m naoh for neutralization.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1



Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
Oxalic acid is a diprotic acid. calculate the percent of oxalic acid (h2c2o4) in a solid given that...
Questions

Mathematics, 14.11.2020 04:50

Mathematics, 14.11.2020 04:50


Business, 14.11.2020 04:50

Chemistry, 14.11.2020 04:50


Mathematics, 14.11.2020 04:50

History, 14.11.2020 04:50

History, 14.11.2020 04:50




English, 14.11.2020 04:50

Mathematics, 14.11.2020 04:50

Business, 14.11.2020 04:50

Mathematics, 14.11.2020 04:50



Arts, 14.11.2020 04:50

Mathematics, 14.11.2020 04:50

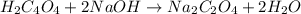
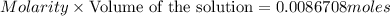
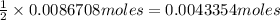 of oxalic acid.
of oxalic acid.