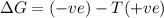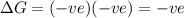Chemistry, 12.10.2019 15:00 GreatBaconGamer
Which of the following circumstances will result in a reaction that is spontaneous at all temperatures?
positive enthalpy change and positive entropy change
negative enthalpy change and negative entropy
change positive enthalpy change and negative entropy change
negative enthalpy change and positive entropy change

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:30
The length of a vector arrow represents its magnitude and the point represents its direction true or false apex
Answers: 3


Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

You know the right answer?
Which of the following circumstances will result in a reaction that is spontaneous at all temperatur...
Questions


Mathematics, 16.03.2020 20:29



















 = Gibbs free energy
= Gibbs free energy 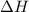 = enthalpy change
= enthalpy change = entropy change
= entropy change