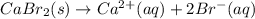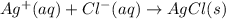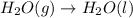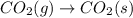Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:30
If a 60-g object has a volume of 30 cm3, what is its density? 2 g/cm3 0.5 cm3/g 1800 g * cm3 none of the above
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

You know the right answer?
For which of the following processes would you expect there to be an increase in entropy? ag+(aq) +...
Questions

Chemistry, 28.04.2021 21:50


Mathematics, 28.04.2021 21:50


Mathematics, 28.04.2021 21:50

Mathematics, 28.04.2021 21:50

Mathematics, 28.04.2021 21:50

Spanish, 28.04.2021 21:50



Biology, 28.04.2021 21:50

Physics, 28.04.2021 21:50


Mathematics, 28.04.2021 21:50

Mathematics, 28.04.2021 21:50

English, 28.04.2021 21:50



Mathematics, 28.04.2021 21:50

Chemistry, 28.04.2021 21:50