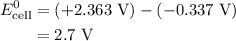Chemistry, 17.09.2019 07:30 nuggetslices
Calculate the standard emf of a cell that uses the mg/mg2+ and cu/cu2+ half-cell reactions at 25 °c. write the equation for the cell reaction that occurs under standard-state conditions and write the line notation for the cell.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 21.06.2019 22:30
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1
You know the right answer?
Calculate the standard emf of a cell that uses the mg/mg2+ and cu/cu2+ half-cell reactions at 25 °c....
Questions


Social Studies, 21.01.2022 15:10

English, 21.01.2022 15:10





Chemistry, 21.01.2022 15:10



Mathematics, 21.01.2022 15:10


History, 21.01.2022 15:10

Mathematics, 21.01.2022 15:10



English, 21.01.2022 15:10


English, 21.01.2022 15:10


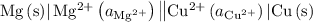
 .
.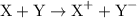
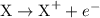

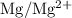 is
is 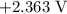 .
.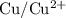 is
is 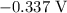 .
.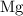 has higher oxidation potential thus the oxidation of
has higher oxidation potential thus the oxidation of 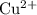 takes place at cathode.
takes place at cathode.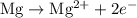 ......(1)
......(1)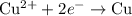 ......(2)
......(2)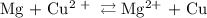 ......(3)
......(3)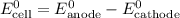 ......(5)
......(5)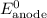 and
and 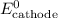 in equation (5).
in equation (5).