Chemistry, 28.09.2019 18:20 ashleyheink3796
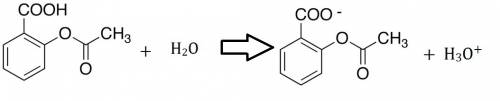
Write a net ionic equation to show that acetylsalicylic acid (aspirin), hc9h7o4, behaves as a brã¸nsted-lowry acid in water.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

You know the right answer?
Write a net ionic equation to show that acetylsalicylic acid (aspirin), hc9h7o4, behaves as a brã¸ns...
Questions

Mathematics, 13.02.2022 05:00

Mathematics, 13.02.2022 05:00

Health, 13.02.2022 05:00

Mathematics, 13.02.2022 05:00



English, 13.02.2022 05:00

Mathematics, 13.02.2022 05:00

History, 13.02.2022 05:00

Mathematics, 13.02.2022 05:00

Mathematics, 13.02.2022 05:00

Mathematics, 13.02.2022 05:00


Social Studies, 13.02.2022 05:00


Mathematics, 13.02.2022 05:00

Mathematics, 13.02.2022 05:00

Health, 13.02.2022 05:00


Mathematics, 13.02.2022 05:00