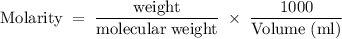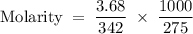Chemistry, 30.09.2019 04:00 mathman783
What is the molarity of a solution that is made by dissolving 3.68 g of sucrose (c12h22o11) in sufficient water to form 275.0 ml of solution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2
You know the right answer?
What is the molarity of a solution that is made by dissolving 3.68 g of sucrose (c12h22o11) in suffi...
Questions


English, 17.02.2022 08:00

Mathematics, 17.02.2022 08:00



Arts, 17.02.2022 08:00



Mathematics, 17.02.2022 08:10

History, 17.02.2022 08:10

SAT, 17.02.2022 08:10

English, 17.02.2022 08:10



Mathematics, 17.02.2022 08:10


History, 17.02.2022 08:10