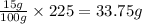Chemistry, 01.09.2019 11:30 hannah2757
Potassium sulfate has a solubility of 15g/100g water at 40 celsius. a solution is prepared by adding 39.0g of potassium sulfate to 225g water, carefully heating the solution, and cooling it to 40 celsius. a homogeneous solution is obtained. is this solution saturated, unsaturated, or supersaturated? the beaker is shaken and precipitation occurs. how many grams of potassium sulfate would you except to crystallize out?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
Potassium sulfate has a solubility of 15g/100g water at 40 celsius. a solution is prepared by adding...
Questions



Biology, 22.12.2020 01:10

Biology, 22.12.2020 01:10

Mathematics, 22.12.2020 01:10


Engineering, 22.12.2020 01:10

Mathematics, 22.12.2020 01:10

English, 22.12.2020 01:10

Business, 22.12.2020 01:10







Mathematics, 22.12.2020 01:10



Physics, 22.12.2020 01:10