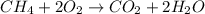Chemistry, 11.10.2019 10:50 rebecca7415
Methane (ch4) is the main component of natural gas. it is burned for fuel in a combustion reaction. the unbalanced combustion reaction for methane is shown below. ch4 + o2 co2 + h2o + heat when the reaction is balanced, how many carbon dioxide molecules are produced for every methane molecule burned?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2


Chemistry, 23.06.2019 07:00
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2
You know the right answer?
Methane (ch4) is the main component of natural gas. it is burned for fuel in a combustion reaction....
Questions

Arts, 24.03.2021 17:40

History, 24.03.2021 17:40

Arts, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40






Computers and Technology, 24.03.2021 17:40



Mathematics, 24.03.2021 17:40

World Languages, 24.03.2021 17:40


Mathematics, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40