
Chemistry, 23.09.2019 14:20 demetriascott20
Iron ore is reduced to pure iron by smelting, during which the iron (iii) oxide in the ore reacts with carbon monoxide gas, like this: fe2o3s + 3cog → 2fes + 3co2g suppose an engineer decides to study the rate of this reaction. she prepares four reaction vessels with 158.8g of solid iron (iii) oxide and 33.9g of carbon monoxide gas each. the volume and temperature of each vessel is shown in the table below. arrange the reaction vessels in decreasing order of initial rate of reaction. in other words, select a "1" next to the vessel in which the engineer can reasonably expect the initial rate of reaction to be highest, a "2" next to the vessel in which the initial rate of reaction would be next highest, and so on. rate of reaction a 2.0l 1100.°c b 2.0l 1200.°c c 4.0l 1100.°c d 4.0l 1000.°c

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

Chemistry, 23.06.2019 07:00
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2
You know the right answer?
Iron ore is reduced to pure iron by smelting, during which the iron (iii) oxide in the ore reacts wi...
Questions

History, 18.09.2019 15:50

Mathematics, 18.09.2019 15:50

Geography, 18.09.2019 15:50



Chemistry, 18.09.2019 15:50


Mathematics, 18.09.2019 15:50


Chemistry, 18.09.2019 15:50


Social Studies, 18.09.2019 15:50


Arts, 18.09.2019 15:50

Computers and Technology, 18.09.2019 15:50

Mathematics, 18.09.2019 15:50


Chemistry, 18.09.2019 15:50


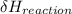 = negative.
= negative.

