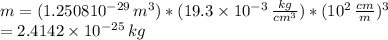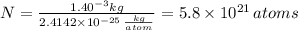Chemistry, 23.09.2019 05:30 robloxlover1987
The atomic radius of a gold atom is 144×10^-12. the volume of a gold atom can be calculated using the volume of a sphere. the density of gold is 19.3 g/cm^3. how many atoms are present in a sample of gold with a mass of 1.40g using the info provided

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1



Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2
You know the right answer?
The atomic radius of a gold atom is 144×10^-12. the volume of a gold atom can be calculated using th...
Questions





Mathematics, 06.07.2021 15:40

Spanish, 06.07.2021 15:40


Computers and Technology, 06.07.2021 15:40

Computers and Technology, 06.07.2021 15:40










Mathematics, 06.07.2021 15:40