

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2
You know the right answer?
As k2o dissolves in water, the oxide ion reacts with water molecules to form hydroxide ions. write t...
Questions

Health, 14.07.2020 17:01



Mathematics, 14.07.2020 17:01





Mathematics, 14.07.2020 17:01

Mathematics, 14.07.2020 17:01

English, 14.07.2020 17:01

Mathematics, 14.07.2020 17:01



Mathematics, 14.07.2020 17:01


Mathematics, 14.07.2020 17:01



Mathematics, 14.07.2020 17:01

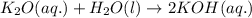
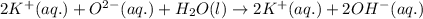
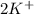 ions are present on both the sides, so the net ionic equation becomes,
ions are present on both the sides, so the net ionic equation becomes,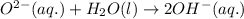
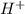 ions.
ions.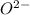 therefore it acts as an acid and oxide ion acts as a base.
therefore it acts as an acid and oxide ion acts as a base.

