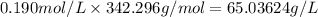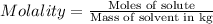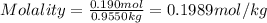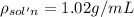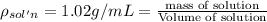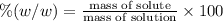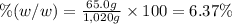Chemistry, 02.10.2019 12:30 irenecupcake4348
Asucrose solution is prepared to a final concentration of 0.190 m . convert this value into terms of g/l, molality, and mass % (molecular weight, mwsucrose = 342.296 g/mol ; density, ρsol′n = 1.02 g/ml ; mass of water, mwat = 955.0 g ). note that the mass of solute is included in the density of the solution.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:10
Which of these is the result of scientific research and not engineering? a. a new shoe design that features air cushioning for more comfort and protection b. the creation of glass with uv protection. c. a conclusion about diet commonalities among diabetics. d. the development of a smaller, more compact missile.
Answers: 1

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1
You know the right answer?
Asucrose solution is prepared to a final concentration of 0.190 m . convert this value into terms of...
Questions

Mathematics, 21.04.2021 23:30

Mathematics, 21.04.2021 23:30





Geography, 21.04.2021 23:30

History, 21.04.2021 23:30

SAT, 21.04.2021 23:30



Mathematics, 21.04.2021 23:30

Mathematics, 21.04.2021 23:30

English, 21.04.2021 23:30

English, 21.04.2021 23:30