

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 07:40
Which of the following has expanded our knowledge of the universe beyond our solar system the most? a. manned space travel b. the hubble space telescope c. the pioneer and voyager missions d. the international space station
Answers: 3
You know the right answer?
What is the limiting reactant when 4.5 moles of aluminum react with 6.7 moles of oxygen gas? unbala...
Questions


English, 26.01.2021 21:50


Mathematics, 26.01.2021 21:50

Chemistry, 26.01.2021 21:50


Mathematics, 26.01.2021 21:50

Biology, 26.01.2021 21:50



Mathematics, 26.01.2021 21:50



Mathematics, 26.01.2021 21:50



Health, 26.01.2021 21:50



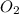 = 6.7 moles
= 6.7 moles
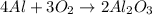
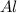 react with 2 moles of
react with 2 moles of 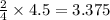 moles of
moles of 

