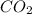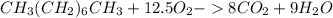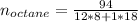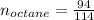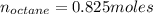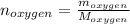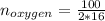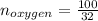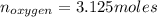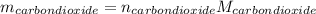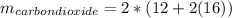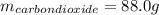Chemistry, 30.08.2019 16:20 deadpoolcorvettehats
Liquid octane ch3ch26ch3 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gaseous water h2o . suppose 94. g of octane is mixed with 100. g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. round your answer to 3 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2


Chemistry, 23.06.2019 04:40
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1

You know the right answer?
Liquid octane ch3ch26ch3 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and...
Questions




Computers and Technology, 09.10.2021 23:00



Mathematics, 09.10.2021 23:00


Social Studies, 09.10.2021 23:00


Mathematics, 09.10.2021 23:00

Physics, 09.10.2021 23:00




Physics, 09.10.2021 23:00

Mathematics, 09.10.2021 23:00


Mathematics, 09.10.2021 23:00