At stp, 1.0 liter of helium contains the same total number of atoms as
(1) 1.0 l of ne (3) 0.5...

Chemistry, 07.10.2019 05:30 dessssimartinez6780
At stp, 1.0 liter of helium contains the same total number of atoms as
(1) 1.0 l of ne (3) 0.5 l of rn
(2) 2.0 l of kr (4) 1.5 l of ar

Answers: 1
Another question on Chemistry

Chemistry, 20.06.2019 18:04
Can anyone (a-level) a student was analysing a carbonate compound, mco3, containing an unknown group 2 metal, m. the student carried out thermal decomposition on 0.730g of the carbonate and measured the volume of gas produced. mco3 (s) mo (s) + co2 (g) the student collected and measured 120cm3 of carbon dioxide. 1 mol of carbon dioxide occupies 24 000 cm3 under these conditions. calculate the molar mass of the group 2 carbonate and hence deduce the identity of the group 2 metal, m.
Answers: 3

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1
You know the right answer?
Questions


Mathematics, 29.09.2019 23:50

Mathematics, 29.09.2019 23:50

Mathematics, 29.09.2019 23:50



Chemistry, 29.09.2019 23:50


History, 29.09.2019 23:50

Computers and Technology, 29.09.2019 23:50

Social Studies, 29.09.2019 23:50




Social Studies, 29.09.2019 23:50

Social Studies, 29.09.2019 23:50



Mathematics, 29.09.2019 23:50

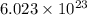 of particles.
of particles.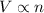 (At constant temperature and pressure)
(At constant temperature and pressure) 

