Given the balanced equation:
2c + 3h2==> c2h6
what is the total number of moles of...

Chemistry, 02.09.2019 08:30 savannahvargas512
Given the balanced equation:
2c + 3h2==> c2h6
what is the total number of moles of c that must completely react to produce 2.0 moles of c2h6?
(1) 1.0 mol (3) 3.0 mol
(2) 2.0 mol (4) 4.0 mol

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
Questions


Mathematics, 12.02.2022 05:30

Advanced Placement (AP), 12.02.2022 05:30

Social Studies, 12.02.2022 05:30




History, 12.02.2022 05:40

Social Studies, 12.02.2022 05:40




Biology, 12.02.2022 05:40



Mathematics, 12.02.2022 05:40

Chemistry, 12.02.2022 05:40



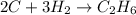
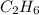 is obtained from 2 moles of C gives
is obtained from 2 moles of C gives moles of C =4 moles
moles of C =4 moles

