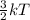Chemistry, 15.01.2020 01:31 braydentillery1221
Asample of gas is enclosed in a container of fixed volume. identify which of the following statements are true. check all that apply. if the container is heated, the gas particles will lose kinetic energy and temperature will increase.
if the container is heated, the gas particles will lose kinetic energy and temperature will increase.
if the container is cooled, the gas particles will lose kinetic energy and temperature will decrease.
if the gas particles move more quickly, they will collide more frequently with the walls of the container and pressure will increase.
if the gas particles move more quickly, they will lose energy more rapidly and collide with the walls of the container less often, and pressure will decrease.
if the gas particles move more quickly, they will collide with the walls of the container more often and with more force, and pressure will increase.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is a character of nuclear fusion but not nuclear fission
Answers: 3

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3
You know the right answer?
Asample of gas is enclosed in a container of fixed volume. identify which of the following statement...
Questions



Mathematics, 17.02.2021 21:40

Mathematics, 17.02.2021 21:40

Mathematics, 17.02.2021 21:40

Mathematics, 17.02.2021 21:40

Mathematics, 17.02.2021 21:40

Physics, 17.02.2021 21:40

Mathematics, 17.02.2021 21:40

English, 17.02.2021 21:40



Mathematics, 17.02.2021 21:40

History, 17.02.2021 21:40



Social Studies, 17.02.2021 21:40