Chemistry, 21.09.2019 15:10 campbelldean
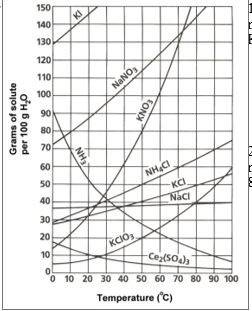
Asaturated solution of nano3 is prepared at 60.°c using 100. grams of water. as this solution is cooled to 10.°c, nano3 precipitates (settles)
out of the solution. the resulting solution is saturated. approximately how many grams of nano3 settled out of the original solution?
(1) 46 g (3) 85 g
(2) 61 g (4) 126 g

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
You know the right answer?
Asaturated solution of nano3 is prepared at 60.°c using 100. grams of water. as this solution is coo...
Questions

Mathematics, 05.03.2021 19:10


Mathematics, 05.03.2021 19:10



Mathematics, 05.03.2021 19:10


Mathematics, 05.03.2021 19:10

Chemistry, 05.03.2021 19:10


Mathematics, 05.03.2021 19:10

Law, 05.03.2021 19:10





Mathematics, 05.03.2021 19:10

Mathematics, 05.03.2021 19:10

Mathematics, 05.03.2021 19:10

Mathematics, 05.03.2021 19:10