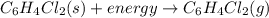Given the balanced equation representing a phase change:
c6h4cl2(s) + energy==> c6h4cl2(g)
which statement describes this change?
(1) it is endothermic, and entropy decreases.
(2) it is endothermic, and entropy increases.
(3) it is exothermic, and entropy decreases.
(4) it is exothermic, and entropy increases.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
Given the balanced equation representing a phase change:
c6h4cl2(s) + energy==> c6h4cl2(g)...
c6h4cl2(s) + energy==> c6h4cl2(g)...
Questions

History, 21.05.2021 19:50



Mathematics, 21.05.2021 19:50



Mathematics, 21.05.2021 19:50

Mathematics, 21.05.2021 19:50

Biology, 21.05.2021 19:50

History, 21.05.2021 19:50


Chemistry, 21.05.2021 19:50

Chemistry, 21.05.2021 19:50


Mathematics, 21.05.2021 19:50

Chemistry, 21.05.2021 19:50

Mathematics, 21.05.2021 19:50


History, 21.05.2021 19:50