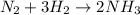Chemistry, 31.08.2019 16:30 icashaypabozp
In 1912, chemist fritz haber developed a process that combined nitrogen from the air with hydrogen at high temperatures and pressures to make ammonia. specifically, the process involved combining one molecule of nitrogen gas (n2) with three molecules of hydrogen gas (h2) to get two molecules of ammonia (nh3). if you write this process in a symbol format, it looks like this:
n2 + 3h2 → 2nh3
explain whether this is a chemical or physical change, and why. does it involve elements, compounds, mixtures, or pure substances? also describe how many atoms are involved before and after. what do you notice about the number of atoms?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1


Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3
You know the right answer?
In 1912, chemist fritz haber developed a process that combined nitrogen from the air with hydrogen a...
Questions




Chemistry, 19.03.2021 21:00





History, 19.03.2021 21:00


Mathematics, 19.03.2021 21:00




Mathematics, 19.03.2021 21:00

Computers and Technology, 19.03.2021 21:00



English, 19.03.2021 21:00

Mathematics, 19.03.2021 21:00