
Chemistry, 24.08.2019 23:20 eeromaki1321
For the reaction:
6li(s)+n2(g)→2li3n(s)
determine:
the mass of n2 needed to react with 0.536 moles of li.
the number of moles of li required to make 46.4 g of li3n.
the mass in grams of li3n produced from 3.65 g li.
the number of moles of lithium needed to react with 7.00 grams of n2.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
For the reaction:
6li(s)+n2(g)→2li3n(s)
determine:
the mass of n2 needed to reac...
6li(s)+n2(g)→2li3n(s)
determine:
the mass of n2 needed to reac...
Questions




History, 09.04.2020 02:20

Mathematics, 09.04.2020 02:20

Mathematics, 09.04.2020 02:20


Physics, 09.04.2020 02:20



Mathematics, 09.04.2020 02:20

Mathematics, 09.04.2020 02:20

Biology, 09.04.2020 02:20


English, 09.04.2020 02:20


Business, 09.04.2020 02:21

Social Studies, 09.04.2020 02:21

Social Studies, 09.04.2020 02:21

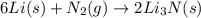
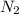 needed to react with 0.536 moles of Li.
needed to react with 0.536 moles of Li.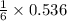 moles of
moles of ![N_2[tex] gas needed:[tex]=28 g/mol\times 0.0893 mol=2.5004 g](/tpl/images/0194/9373/6ce3e.png)
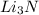
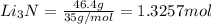
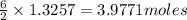
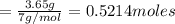
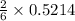 that is 0.1738 moles of
that is 0.1738 moles of 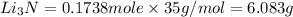
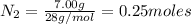
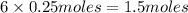 of lithium
of lithium

