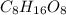Chemistry, 25.09.2019 15:00 liferoblox666
Acompound is 40.0% c, 6.70% h, and 53.3% o by mass. assume that we have a 100.-g sample of this compound. the molecular formula mass of this compound is 240 amu . what are the subscripts in the actual molecular formula?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
Acompound is 40.0% c, 6.70% h, and 53.3% o by mass. assume that we have a 100.-g sample of this comp...
Questions

Mathematics, 20.09.2020 01:01

Engineering, 20.09.2020 01:01

English, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01

English, 20.09.2020 01:01




Mathematics, 20.09.2020 01:01



Mathematics, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01

Social Studies, 20.09.2020 01:01


Geography, 20.09.2020 01:01




Mathematics, 20.09.2020 01:01