Chemistry, 19.12.2019 13:31 dakshshberry
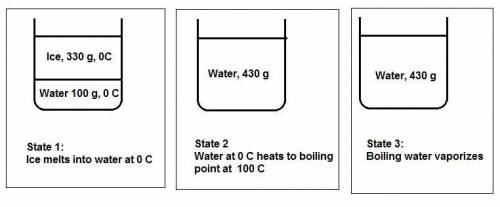
Water's heat of fusion is 80. cal/g , its specific heat is 1.0calg⋅∘c, and its heat of vaporization is 540 cal/g . a canister is filled with 330 g of ice and 100. g of liquid water, both at 0 ∘c . the canister is placed in an oven until all the h2o has boiled off and the canister is empty. how much energy in calories was absorbed?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 08:30
7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation. 8. when a 2.5 mol of sugar (c12h22o11) are added to a certain amount of water the boiling point is raised by 1 celsius degree. if 2.5 mol of aluminum nitrate is added to the same amount of water, by how much will the boiling point be changed? show all calculations leading to your answer or use 3 – 4 sentences to explain your answer. 9. if 5.40 kcal of heat is added to 1.00 kg of water at 100⁰c, how much steam at 100⁰c is produced? show all calculations leading to an answer. 10. the freezing of water at 0⁰c can be represented as follows: h2o (l) ↔ h2o(s) the density of liquid water is 1.00 g/cm3. the density of ice is 0.92 g/cm3. in 3 – 4 sentences explain why applying pressure causes ice to melt.
Answers: 1
You know the right answer?
Water's heat of fusion is 80. cal/g , its specific heat is 1.0calg⋅∘c, and its heat of vaporization...
Questions

English, 06.08.2019 16:10

English, 06.08.2019 16:10

English, 06.08.2019 16:10




Chemistry, 06.08.2019 16:10

Mathematics, 06.08.2019 16:10



Mathematics, 06.08.2019 16:10




English, 06.08.2019 16:10


English, 06.08.2019 16:10