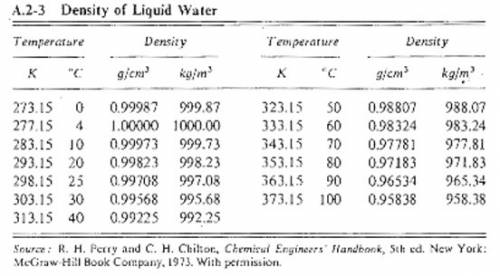
Suppose you were calibrating a 100.0 ml volumetric flask using distilled water. the flask temperature was at 20°c, and you assumed that the distilled water was as well. however, you later discover that the actual water temperature was 11°c instead. how is the mass of the 100.0 ml of distilled water you measured at 11°c different from the mass of 100.0 ml of distilled water at 20°c?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1

Chemistry, 23.06.2019 15:00
How many more valence electrons does sodium need to have a full outer valence shell
Answers: 3
You know the right answer?
Suppose you were calibrating a 100.0 ml volumetric flask using distilled water. the flask temperatur...
Questions

Mathematics, 24.08.2019 22:50


Computers and Technology, 24.08.2019 22:50




Mathematics, 24.08.2019 22:50

Social Studies, 24.08.2019 22:50

Mathematics, 24.08.2019 22:50

Biology, 24.08.2019 22:50


Social Studies, 24.08.2019 22:50

Mathematics, 24.08.2019 22:50


Mathematics, 24.08.2019 22:50

Mathematics, 24.08.2019 22:50


History, 24.08.2019 22:50