Chemistry, 29.09.2019 18:30 andrejr0330jr
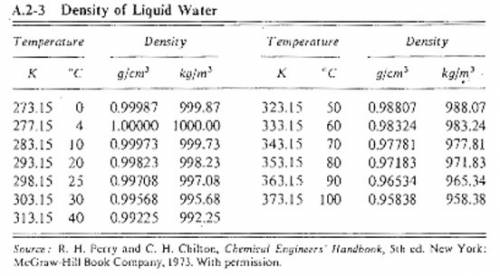
Suppose you were calibrating a 50.0 ml volumetric flask using distilled water. the flask temperature was at 20°c, and you assumed that the distilled water was as well. however, you later discover that the actual water temperature was 14°c instead. how is the mass of the 50.0 ml of distilled water you measured at 14°c different from the mass of 50.0 ml of distilled water at 20°c?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2
You know the right answer?
Suppose you were calibrating a 50.0 ml volumetric flask using distilled water. the flask temperature...
Questions


Mathematics, 06.12.2019 00:31

Social Studies, 06.12.2019 00:31


English, 06.12.2019 00:31

Mathematics, 06.12.2019 00:31





Chemistry, 06.12.2019 00:31




Mathematics, 06.12.2019 00:31


Mathematics, 06.12.2019 00:31