
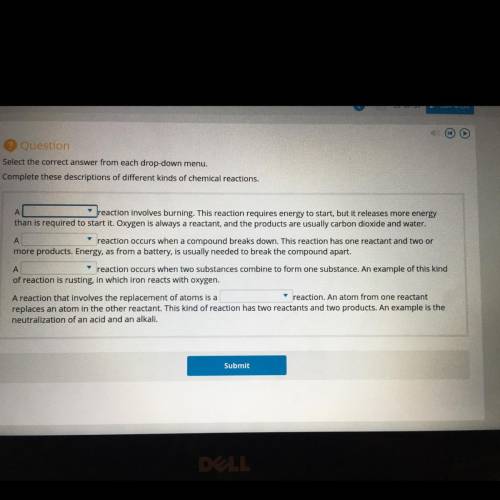
Reaction involves burning. This reaction requires energy to start, but it releases more energy
than is required to start it. Oxygen is always a reactant, and the products are usually carbon dioxide and water,
A
reaction occurs when a compound breaks down. This reaction has one reactant and two or
more products, Energy, as from a battery, is usually needed to break the compound apart.
A
reaction occurs when two substances combine to form one substance. An example of this kind
of reaction is rusting, in which iron reacts with oxygen.
A reaction that involves the replacement of atoms is a
reaction. An atom from one reactant
replaces an atom in the other reactant. This kind of reaction has two reactants and two products. An example is the
neutralization of an acid and an alkali.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1
You know the right answer?
Reaction involves burning. This reaction requires energy to start, but it releases more energy
tha...
Questions

Chemistry, 20.10.2021 06:20



Mathematics, 20.10.2021 06:20




Mathematics, 20.10.2021 06:20


Mathematics, 20.10.2021 06:20

Biology, 20.10.2021 06:20


Computers and Technology, 20.10.2021 06:20

English, 20.10.2021 06:20


History, 20.10.2021 06:20

Chemistry, 20.10.2021 06:20


Mathematics, 20.10.2021 06:20



