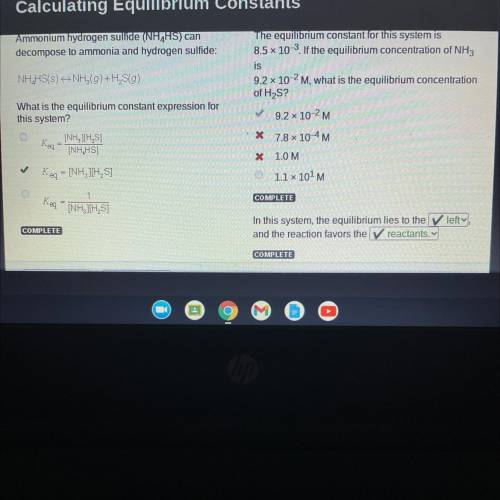
Ammonium hydrogen sulfide (NH4HS) can
decompose to ammonia and hydrogen sulfide:
NH4HS(s)<...

Chemistry, 20.04.2021 18:50 Loveekatiana
Ammonium hydrogen sulfide (NH4HS) can
decompose to ammonia and hydrogen sulfide:
NH4HS(s)<—>NH3(g)+ H2S(g)
What is the equilibrium constant expression for
this system?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1

Chemistry, 23.06.2019 16:00
When electrons are removed from the outermost shell of a calcium atom, the atom becomes an anion that has a larger radius than the atom. an anion that has a smaller radius than the atom. a cation that has a larger radius than the atom. a cation that has a smaller radius than the atom.
Answers: 3

Chemistry, 23.06.2019 18:40
Sound does not need a medium to travel is reflected when it bounces off a shiny surface is created by electric and magnetic fields moves in longitudinal waves
Answers: 1
You know the right answer?
Questions



Chemistry, 02.12.2020 17:10

Mathematics, 02.12.2020 17:10

Mathematics, 02.12.2020 17:10


Mathematics, 02.12.2020 17:10




Business, 02.12.2020 17:10

Mathematics, 02.12.2020 17:10

Mathematics, 02.12.2020 17:10

Biology, 02.12.2020 17:10



Mathematics, 02.12.2020 17:10

History, 02.12.2020 17:10

Physics, 02.12.2020 17:10



