
Chemistry, 20.04.2021 22:40 alyssaboosiefkes
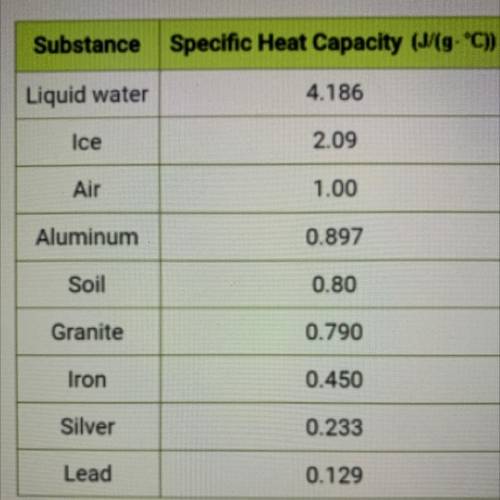
Use the specific heat value from the table at left to calculate the amount
of energy in Joules required to raise the temperature of 5.08 g of
aluminum by 13.20 °C. Do not record units in your answer. Round to
the tenths place or further.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1


Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
You know the right answer?
Use the specific heat value from the table at left to calculate the amount
of energy in Joules req...
Questions


Biology, 22.03.2021 16:40



Physics, 22.03.2021 16:40

Chemistry, 22.03.2021 16:40

Mathematics, 22.03.2021 16:40

Computers and Technology, 22.03.2021 16:40



Mathematics, 22.03.2021 16:40



English, 22.03.2021 16:40

Spanish, 22.03.2021 16:40


Mathematics, 22.03.2021 16:40





