Ok, i need a help.
Knowing that the initial concentration of phosphoric acid
within a pool i...

Chemistry, 21.04.2021 06:50 tabisulaprinces2084
Ok, i need a help.
Knowing that the initial concentration of phosphoric acid
within a pool is 0.3mg. L-¹, and considering the equilibrium constants and the equilibrium expressions placed in the figure, determine the concentration of all specific species in the solution.
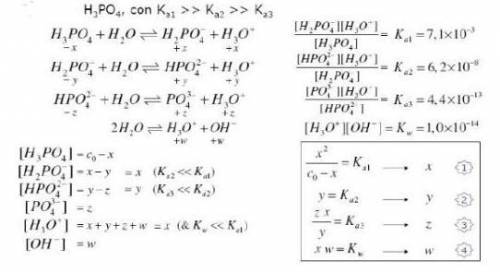

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2
You know the right answer?
Questions

Biology, 22.03.2021 17:50


Mathematics, 22.03.2021 17:50

Mathematics, 22.03.2021 17:50





Chemistry, 22.03.2021 17:50


World Languages, 22.03.2021 17:50

Mathematics, 22.03.2021 17:50

Health, 22.03.2021 17:50

Mathematics, 22.03.2021 17:50


Mathematics, 22.03.2021 17:50

Chemistry, 22.03.2021 17:50

Mathematics, 22.03.2021 17:50

Mathematics, 22.03.2021 17:50

History, 22.03.2021 17:50



