
Chemistry, 21.04.2021 07:10 minelly1717
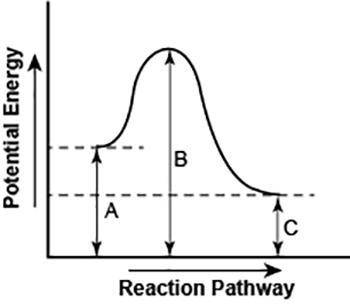
Part 1: Looking at the diagram above, what can you tell me about the type of reaction it is? Is this exothermic or endothermic? How do you know? Make sure you support your answer by using the diagram.
Part 2: Thinking about enthalpy and a change in enthalpy, explain how you could find the total change of enthalpy based on this diagram. Is the enthalpy positive or negative?
Part 3: How could you find the activation energy? Is the activation energy positive or negative?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl+4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1


Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2
You know the right answer?
Part 1: Looking at the diagram above, what can you tell me about the type of reaction it is? Is this...
Questions



History, 06.10.2021 17:10


Physics, 06.10.2021 17:10


English, 06.10.2021 17:10

Mathematics, 06.10.2021 17:10

Physics, 06.10.2021 17:10

English, 06.10.2021 17:10

Mathematics, 06.10.2021 17:10




Chemistry, 06.10.2021 17:10



Mathematics, 06.10.2021 17:10

Computers and Technology, 06.10.2021 17:10

English, 06.10.2021 17:20



