Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1
You know the right answer?
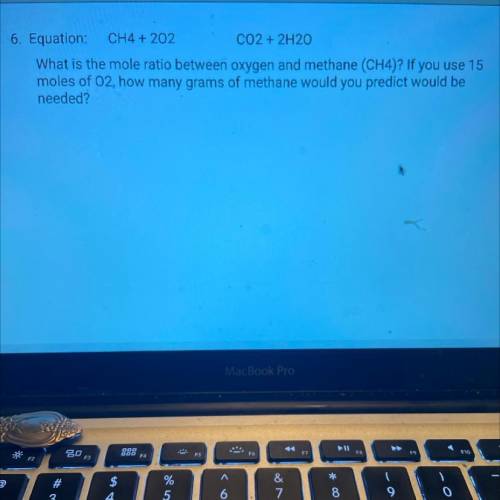
Equation: CH4 + 202 CO2 + 2H20
What is the mole ratio between oxygen and methane (CH4)? If you use...
Questions

Business, 14.01.2020 05:31


Biology, 14.01.2020 05:31

Computers and Technology, 14.01.2020 05:31


English, 14.01.2020 05:31



Social Studies, 14.01.2020 05:31


Social Studies, 14.01.2020 05:31

Social Studies, 14.01.2020 05:31








History, 14.01.2020 05:31