Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
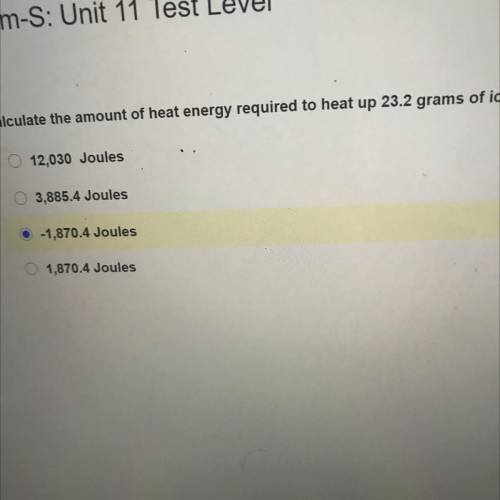
Chem-S: Unit 11 Test Level
Calculate the amount of heat energy required to heat up 23.2 grams of i...
Questions




Mathematics, 16.12.2019 10:31

World Languages, 16.12.2019 10:31


Mathematics, 16.12.2019 10:31

Mathematics, 16.12.2019 10:31

Social Studies, 16.12.2019 10:31

Mathematics, 16.12.2019 10:31

History, 16.12.2019 10:31



Chemistry, 16.12.2019 10:31




Mathematics, 16.12.2019 10:31