
Chemistry, 21.04.2021 23:00 chanahvanya
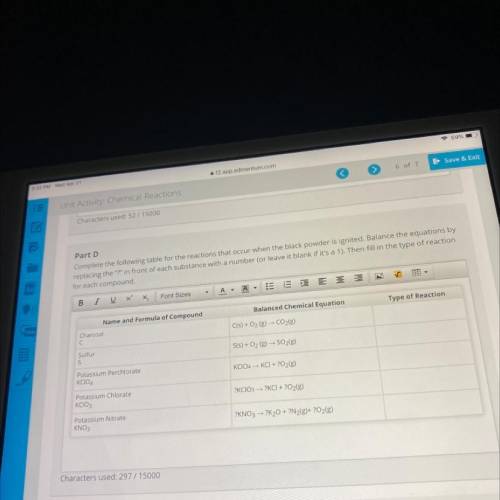
Part D
Complete the following table for the reactions that occur when the black powder is ignited. Balance the equations by
replacing the "?" in front of each substance with a number (or leave it blank if it's a 1). Then fill in the type of reaction
for each compound.
B.
I X
X
Font Sizes
A-
A-
= = 三 三 三
A
Name and Formula of Compound
Balanced Chemical Equation
Type of Reaction
Charcoal
C
C(s) + O2 (g) - CO2(g)
Sulfur
S
S(s) + O2(g) → SO2(g)
Potassium Perchlorate
KCIO4
KCIO4 - KCI + O2(g)
Potassium Chlorate
KCIO3
?KCIO3 → ?KCI + 2O2(g)
Potassium Nitrate
KNO3
?KNO3 → ?K20 + ?N2(g)+ ?O2(g)
Characters used: 297/ 15000

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
Part D
Complete the following table for the reactions that occur when the black powder is ignited....
Questions


Mathematics, 28.06.2020 15:01

Health, 28.06.2020 15:01

Mathematics, 28.06.2020 15:01

Mathematics, 28.06.2020 15:01


Mathematics, 28.06.2020 15:01



Mathematics, 28.06.2020 15:01

English, 28.06.2020 15:01

Social Studies, 28.06.2020 15:01

Mathematics, 28.06.2020 15:01

Law, 28.06.2020 15:01


Chemistry, 28.06.2020 15:01



Geography, 28.06.2020 15:01



