
Chemistry, 22.04.2021 01:20 emmanuel180
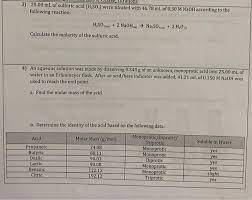
An aqueous solution was made by dissolving 0.543 g of an unknown, monoprotic acid into 25.00 mL of water in an Erlenmeyer flask. After an acid/base indicator was added, 41.21 mL of 0.150 M NaOH was used to reach the end point.
a)Find the molar mass of the acid.
b)Determine the identity of the acid based on the following data:
PLEASE HELP ME

Answers: 3
Another question on Chemistry

Chemistry, 20.06.2019 18:02
Which is an example of renewableenergy resource uranium ,wind, or natural gas
Answers: 2

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

You know the right answer?
An aqueous solution was made by dissolving 0.543 g of an unknown, monoprotic acid into 25.00 mL of w...
Questions



Chemistry, 10.02.2020 03:32







Mathematics, 10.02.2020 03:33

Mathematics, 10.02.2020 03:33

History, 10.02.2020 03:34

Social Studies, 10.02.2020 03:34




Biology, 10.02.2020 03:34

Health, 10.02.2020 03:34




