
Chemistry, 22.04.2021 18:20 brennae8529
If the formula of ammonium nitrate is NH4NO3, determine how many moles of the solid were dissolved in the water in the experiment #2.
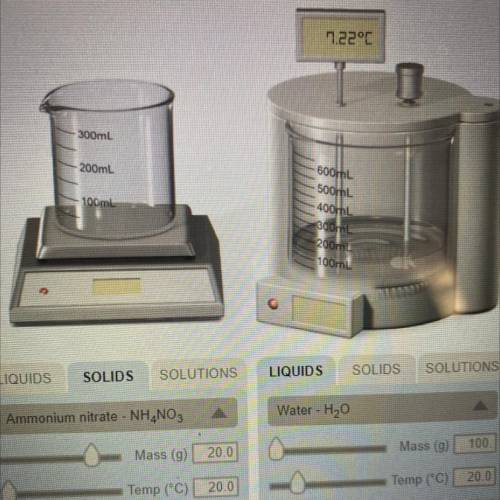

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
If the formula of ammonium nitrate is NH4NO3, determine how many moles of the solid were dissolved i...
Questions


History, 07.12.2020 01:20

English, 07.12.2020 01:20

Mathematics, 07.12.2020 01:20

Chemistry, 07.12.2020 01:20




Chemistry, 07.12.2020 01:20




Biology, 07.12.2020 01:20

Physics, 07.12.2020 01:20

Mathematics, 07.12.2020 01:20

History, 07.12.2020 01:20

English, 07.12.2020 01:20

Mathematics, 07.12.2020 01:20

Arts, 07.12.2020 01:20

Geography, 07.12.2020 01:20



