
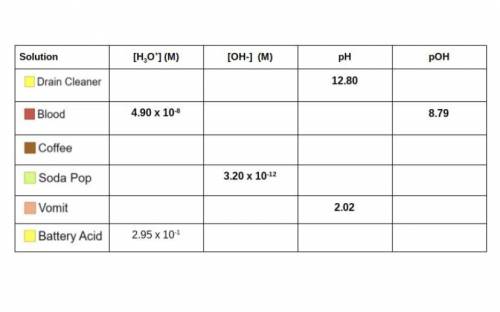
Use your knowledge of Molarity, pH/pOH, and the concentrations of ions to complete the table below. Some of
the work can be checked using the simulation. I have included a handy flow chart below the table with
common conversions when dealing with acids and bases. You will need to know how to do these calculations
for the unit test! (Picture attached)

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2
You know the right answer?
Use your knowledge of Molarity, pH/pOH, and the concentrations of ions to complete the table below....
Questions


Mathematics, 19.10.2019 18:30


History, 19.10.2019 18:30

English, 19.10.2019 18:30

Mathematics, 19.10.2019 18:30

Mathematics, 19.10.2019 18:30


Biology, 19.10.2019 18:30

Mathematics, 19.10.2019 18:30

Geography, 19.10.2019 18:30




History, 19.10.2019 18:30


Mathematics, 19.10.2019 18:30

Physics, 19.10.2019 18:30

Mathematics, 19.10.2019 18:30

English, 19.10.2019 18:30



