
Chemistry, 23.04.2021 17:10 esuqugip9498
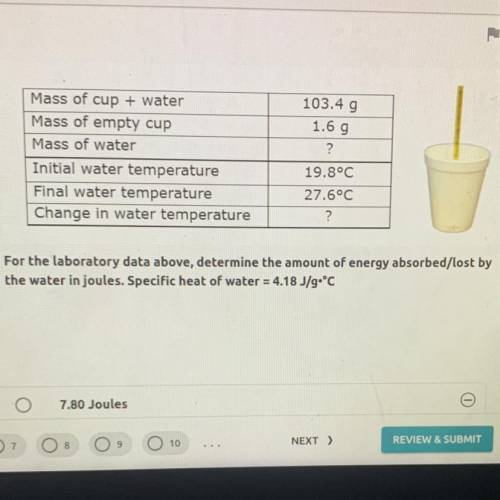
For the laboratory data above, determine the amount of energy absorbed/lost by the water in joules. Specific heat of water = 4.18 J/g•°C

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2


Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
For the laboratory data above, determine the amount of energy absorbed/lost by
the water in joules...
Questions

Mathematics, 01.04.2020 20:54

Chemistry, 01.04.2020 20:54

History, 01.04.2020 20:54



Mathematics, 01.04.2020 20:54

Mathematics, 01.04.2020 20:54



Biology, 01.04.2020 20:54







History, 01.04.2020 20:54


Biology, 01.04.2020 20:54



