
Chemistry, 23.04.2021 18:40 WilliamYES9164
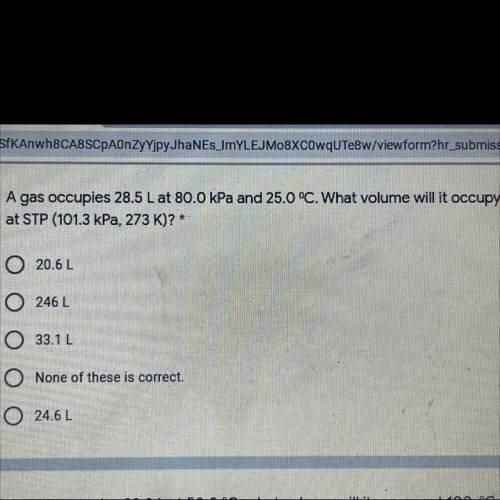
A gas occupies 28.5 L at 80.0 kPa and 25.0 °C. What volume will it occupy
at STP (101.3 kPa, 273 K)? *
A. 20.6L
B. 246 L
C. 33.12
D. None of these is correct.
E. 24.62

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
A gas occupies 28.5 L at 80.0 kPa and 25.0 °C. What volume will it occupy
at STP (101.3 kPa, 273...
Questions

Social Studies, 10.02.2021 21:30






Mathematics, 10.02.2021 21:30


Health, 10.02.2021 21:30

Social Studies, 10.02.2021 21:30




English, 10.02.2021 21:30

Mathematics, 10.02.2021 21:30

History, 10.02.2021 21:30

Mathematics, 10.02.2021 21:30

Advanced Placement (AP), 10.02.2021 21:30

Mathematics, 10.02.2021 21:30

History, 10.02.2021 21:30



