
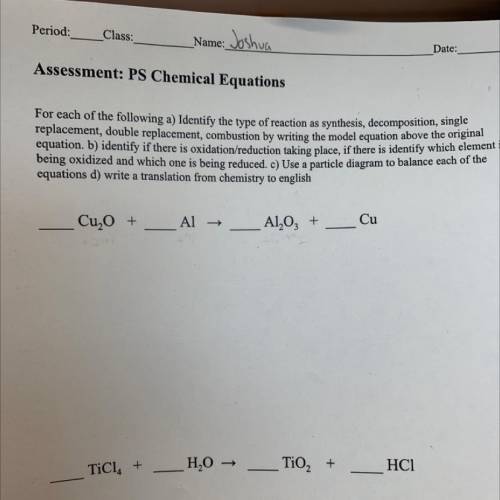
Assessment: PS Chemical Equations
For each of the following a) Identify the type of reaction as synthesis, decomposition, single
replacement, double replacement, combustion by writing the model equation above the original
equation, b) identify if there is oxidation/reduction
taking place, if there is identify which element i
being oxidized and which
one is being reduced. C) Use a particle diagram to balance each of the
equations d) write a translation from chemistry to english
Cu, O +
Al →
Al2O3 +
Cu
TiCl. +
H2O →
TiO2 +
HC1

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Theoretically, which metal should be the most reactive? hydrogen lithium francium fluorine
Answers: 1

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1


You know the right answer?
Assessment: PS Chemical Equations
For each of the following a) Identify the type of reaction as sy...
Questions

Mathematics, 02.03.2021 06:00




Biology, 02.03.2021 06:00

English, 02.03.2021 06:00


Mathematics, 02.03.2021 06:00

Mathematics, 02.03.2021 06:00



Mathematics, 02.03.2021 06:00



Mathematics, 02.03.2021 06:00


Mathematics, 02.03.2021 06:00



Mathematics, 02.03.2021 06:00



