
Chemistry, 23.04.2021 22:40 ibarral37102
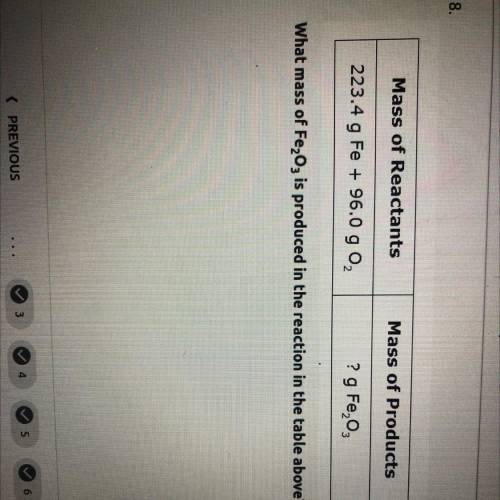
what mass of Fe2O3 is produced in the reaction in the table above mass of reactants= 223.4 g Fe+96.0 g O2. and mass of products=? g Fe2O3

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

You know the right answer?
what mass of Fe2O3 is produced in the reaction in the table above mass of reactants= 223.4 g Fe+96.0...
Questions


Mathematics, 19.03.2021 18:10


Social Studies, 19.03.2021 18:10

Mathematics, 19.03.2021 18:10


Mathematics, 19.03.2021 18:10

Physics, 19.03.2021 18:10

Computers and Technology, 19.03.2021 18:10




History, 19.03.2021 18:10



Computers and Technology, 19.03.2021 18:10


Biology, 19.03.2021 18:10


Social Studies, 19.03.2021 18:10



