Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
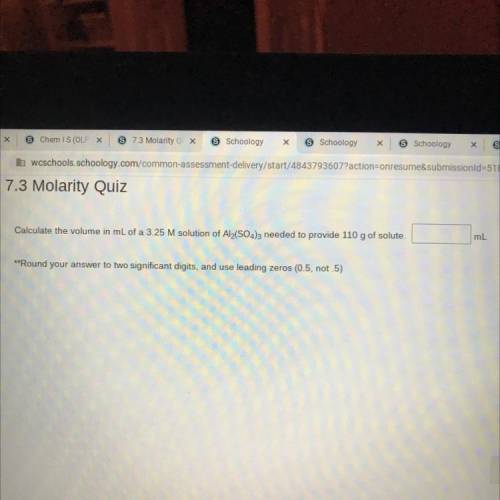
Calculate the volume in mL of a 3.25 M solution of Al2(SO4)3 needed to provide 110 g of solute.
Questions

English, 19.04.2021 14:00


Mathematics, 19.04.2021 14:00

English, 19.04.2021 14:00

English, 19.04.2021 14:00

Mathematics, 19.04.2021 14:00

Mathematics, 19.04.2021 14:00

English, 19.04.2021 14:00


Mathematics, 19.04.2021 14:00


Mathematics, 19.04.2021 14:00


Chemistry, 19.04.2021 14:00

Mathematics, 19.04.2021 14:00

Mathematics, 19.04.2021 14:00

Mathematics, 19.04.2021 14:00

Mathematics, 19.04.2021 14:00