
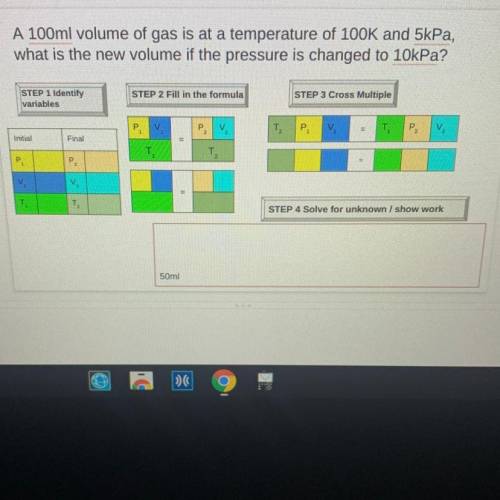
A 100ml volume of gas is at a temperature of 100K and 5kPa,
what is the new volume if the pressure is changed to 10kPa?
STEP 1 Identify
variables
STEP 2 Fill in the formula
STEP 3 Cross Multiple
P
V
T2
P
P2
Initial
Final
P.
P
=
V
V
T
TO
STEP 4 Solve for unknown / show work

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
A 100ml volume of gas is at a temperature of 100K and 5kPa,
what is the new volume if the pressure...
Questions





Mathematics, 05.03.2021 16:50

Social Studies, 05.03.2021 16:50

Mathematics, 05.03.2021 16:50


Mathematics, 05.03.2021 16:50

Mathematics, 05.03.2021 16:50




Mathematics, 05.03.2021 16:50

History, 05.03.2021 16:50



Mathematics, 05.03.2021 16:50




